Introduction
If you are a beginner student struggling with your Organic Chemistry lab and didn’t understand a thing from the tedious lecture given by your professors, don’t worry. Thanks to this article and the materials I provide (downloadable PDFs, video tutorials, and a complete course on Organic Chemistry lab), you’ll surely understand much more. In this article, we specifically talk about TLC (Thin Layer Chromatography), a very common, simple, and useful technique.
Definition
TLC is thin layer chromatography. Therefore, to understand what TLC is, it is necessary to understand the meaning of the two key words that compose it: chromatography and thin layer.
Def. Chromatography
Chromatography is a technique for purifying a mixture of substances by their differential interaction with a mobile phase (liquid, gas, or fluid) and a stationary phase (solid or liquid) placed on a suitable support.
From the definition, it is clearly understood that chromatography is used for purifying compounds. Less clear might be how this purification is achieved. We will clarify this point further when, later in the article, we discuss how TLC works; for now, suffice it to say that in chromatography, two phases are always required for purification: the stationary phase and the mobile phase.
Would you like to read our step-by-step guide on how to perform TLC in the laboratory? You can download the PDF or DOCX file which summarizes both theory and practice on TLC. You’ll find the file at the end of this article.
Classification
There are various types of chromatography, which is why several classifications have been created to rationalize this variety. For example, there is a classification based on the type of mobile phase used (e.g., gas chromatography, where the mobile phase is a gas, or liquid chromatography, where the mobile phase is a liquid). Additionally, there is a classification based on the type of interaction between solute and stationary phase (e.g., adsorption chromatography, partition chromatography, ion exchange chromatography, molecular sieve chromatography).
TLC is part of liquid chromatography because it uses a mixture of solvents as the mobile phase, and it also falls under adsorption chromatography.
The term adsorption chromatography refers to a chromatography where the solute-stationary phase interaction occurs at the surface of the stationary phase through various interactions (hydrogen bonds, dipole-dipole interactions, van der Waals interactions). The word adsorption should not be confused with absorption; the former refers to an interaction solely with the surface of a substance, while the latter refers to an interaction with the entire volume of a substance.
The adsorption chromatography is divided into two further categories depending on the support used for the stationary phase:
Column chromatography, where the stationary phase is packed inside a glass column;
Thin layer chromatography, where the stationary phase is deposited as a thin layer on a glass, aluminum, or plastic support.
So we have finally understood the two key words that make up the name TLC, namely chromatography and thin layer. Let’s now move on to see how TLC is made of physically.
TLC
How is a TLC made?
So, what is a TLC made of? TLC is a plate on which the adsorbent material (the stationary phase) is deposited, often mixed with a binder (such as gypsum, soluble starch, etc.) to provide compactness to the material. In addition to the binder, the stationary phase is often also mixed with a fluorescent indicator that will be used during TLC detection. The stationary phase is thus deposited, along with other materials, on a thin support which can be glass, aluminum, or plastic.
For an example of TLC, you can see the figure below, which shows a 20 x 20 cm TLC on a glass support.
From the description of a TLC, it’s immediately clear that various models of TLC are available on the market, differing in size, type of stationary phase, and support type.
In particular, in addition to the 20×20 cm TLC, there are smaller TLCs, for example, 5×10 cm in size. The ideal size depends on the intended use. For example, the 20×20 cm TLC is used to purify small quantities of sample directly on the plate (20-50 mg), or alternatively, it can be cut to obtain smaller TLC plates. The 5×10 cm TLC, on the other hand, is usually employed for analytical purposes.
However, what should primarily guide the choice of a TLC is the stationary phase, as the quality of compound separation depends on it. There are various stationary phases, but the most common ones are certainly silica gel, alumina, florisil, cellulose, and starch.
How does a TLC work
After seeing how a TLC is made, the next step is to understand how it works.
TLC is performed through the following steps:
1) Apply the sample, appropriately diluted in a solvent, to the loading zone of the TLC. This zone is a line previously drawn with a pencil about 7-8 mm from the bottom of the TLC. The sample is applied using capillaries, which are small diameter glass tubes that collect liquids by capillarity. Loading the sample should be done in a way that creates a small circle with a diameter not exceeding 2 mm.
2) Place the TLC in the elution chamber and add the eluent mixture. The elution chamber is a container that can be sealed at the top with a lid (e.g., a jar). In this chamber, the TLC is inserted along with a solvent mixture, which constitutes the mobile phase (also called the eluent).
3) Elute the TLC. Once the eluent is added and the chamber is sealed with the lid, wait for the solvent to rise by capillarity along the entire TLC. During this phase, the sample will migrate along with the eluent, but its migration rate may vary. Indeed, the sample will migrate very slowly the more it interacts with the stationary phase, while it will migrate more rapidly the less it interacts with the stationary phase. Additionally, if the sample applied on the TLC consists of a mixture of compounds, they will separate during the elution phase because they will migrate at different speeds depending on their affinity for the stationary phase.
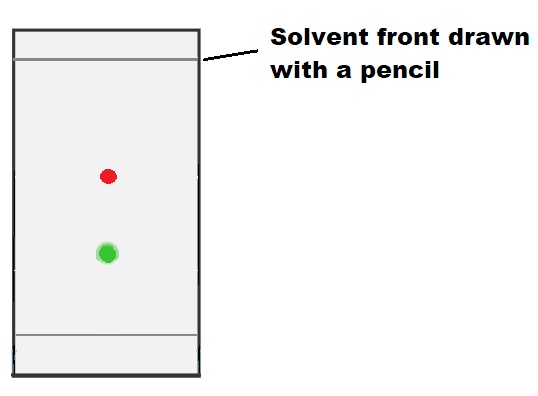
4) Remove the TLC from the elution chamber when the solvent is a few millimeters away from the edge of the TLC, mark the solvent front with a pencil, and reveal the TLC using an appropriate technique..
The images below clarify the steps described above; you just need to scroll through the images to see them all.
Stationary phases
As you may have noticed, TLC cannot function without the stationary phase (or adsorbent) and the mobile phase (or eluent).
In this short paragraph and the following one, we will clarify which stationary phases and eluent mixtures exist and how to choose them.
The most common stationary phases can be classified based on their adsorption power as follows:
Strong adsorption power: alumina, florisil, silica gel, activated charcoal;
Medium adsorption power: calcium hydroxide, magnesium hydroxide, calcium phosphate, calcium carbonate..
Low adsorption power: cellulose, starch, sucrose, talc..
Alumina, aluminum oxide (Al2O3), is the stationary phase with the highest adsorption power and is commercially available in basic, neutral, and acidic forms. It is suitable for separating compounds of medium and low polarity, while it is not recommended for highly polar compounds, such as carboxylic acids, because, due to the high adsorption power of alumina, these compounds could irreversibly bind to it. Among the listed stationary phases, the most commonly used in organic chemistry is certainly silica gel, a polymer of silicon dioxide (SiO2)n. This stationary phase is used to separate a wide range of organic compounds including esters, amines, carbonyl compounds, alcohols, thiols, etc.
The above-listed stationary phases are all polar, which is why they will retain polar organic substances more effectively. In the image below, organic compounds are listed in order of increasing affinity for polar stationary phases.

Compounds with higher affinity for the polar stationary phase will migrate less along the TLC (Thin Layer Chromatography), so we will find them as lower spots. Conversely, less polar substances are poorly retained and will migrate more quickly with the mobile phase, therefore, they will be eluted first.
Eluent mixtures
The eluent mixture consists of one or more solvents with different polarities. The most suitable eluent mixture for our TLC will be the one that allows for good separation of the spots. Typically, the eluent mixture consists of a non-polar solvent (e.g., petroleum ether, hexane, cyclohexane) and a more polar solvent (ethyl acetate, diethyl ether, acetone, etc.). One of the most well-known mixtures to start with when searching for the appropriate eluent mixture is hexane/ethyl acetate in a ratio of 8:2. Various other combinations can be tried by varying the ratio of the two solvents or by changing the solvents themselves. It should be noted, however, that an increase in a polar solvent will result in greater migration of polar organic substances, while an eluent mixture with higher quantities of non-polar solvent leads to greater retention of more polar spots.
An aid in choosing the eluent mixture can be provided by the eluotropic series, a classification of solvents in increasing order of eluting power (see the figure below).

Purposes
After seeing how TLC works and what the stationary phase and mobile phase are, it’s time to clarify when exactly TLC is used in the lab.
Below are listed the most common applications.
Search for the suitable eluent for column chromatography. Column chromatography is used to purify large quantities of sample and consumes a lot of solvent. Its operating principle is similar to TLC, which is why small-scale tests are conducted using TLC to find the appropriate eluent mixture for separation with column chromatography.
Monitoring the progress of a chromatographic column. During the chromatographic column run, it’s necessary to perform many TLCs to understand which eluted fractions from the column contain the desired product and if it is pure.
Seguire lo svolgimento di una reazione. Durante una reazione è possibile effetture delle TLC di piccole aliquote prelevate dal pallone di reazione per capire se la reazione è conclusa e ha generato il prodotto di interesse.
Checking the purity of a compound. If a compound is loaded onto TLC and after elution only one band is obtained, this generally implies that the compound is pure.
Recognition of unknown products. Often by spotting unknown and reference products on TLC, one can determine if the unknown sample matches the reference sample.
Detection methods
We haven’t yet clarified how to visualize the spots on TLC after elution. These spots, except for colored compounds, are not visible to the naked eye. An external medium is required to see them. The most commonly used techniques are listed below.
UV lamp. This is the preferred method for visualizing TLC, and only if this does not work, other methods are generally used. The UV lamp for TLC detection is equipped with two wavelengths: 254 and 366 nm. When the wavelength of 254 nm is activated, it is possible to visualize the spots that absorb in UV as dark spots. This occurs because TLC plates are impregnated with a fluorescent indicator that emits green light at this wavelength. However, in areas where samples that absorb in UV are present, such green light does not appear, and instead, the samples will be seen as dark spots. Conversely, at the wavelength of 366 nm, the indicator is no longer able to emit green light, and therefore, all organic compounds that exhibit fluorescence can be seen. Thus, at 254 nm, all organic compounds capable of absorbing in UV (aromatic compounds, conjugates, etc.) will be visible, while at 366 nm, those that exhibit fluorescence will be visible.
Universal method. This method works with all organic compounds and involves spraying the TLC plate, after elution, with concentrated sulfuric acid. The plate is then placed in an oven at 110-120°C. Under these conditions, all substances present on the TLC plate will be oxidized and carbonized, resulting in visible dark spots.
Use of chromogenic reagents. There are various solutions of colored substances (chromogens) that can be prepared and in which TLC plates can be immersed. After immersion, the TLC plates are dried, and the compounds present are revealed as colored bands. There are also chromogens suitable for the detection of specific functional groups. For example, ninhydrin is suitable for the detection of amino acids, bromocresol for organic acids, etc..
Conclusion
In this article, we have covered the most important theoretical aspects of TLC.
If you would like to delve deeper into TLC, we offer you two options:
1) Download the PDF or DOCX file below. This comprehensive document includes a step-by-step guide on performing TLC in the lab, a theoretical summary of TLC, calculation of RF, description of 2D TLC and even a troubleshooting section.
2) Join our course about Organic Chemistry Lab Technique where you can gain comprehensive knowledge about the most common lab practises. Each lessons includes video tutorials, theoretical explanations, downloadable support files, and quizzes. Take a look here: Organic Chemistry Lab Techniques.
Please remember to rate this post; your feedback will assist us in enhancing our services for you.
This lesson

TLC-summary and practical guide
€
2.50
Download as pdf (unchageable) file

TLC-summary and practical guide
€
3.50
Downoald as docx (editable) file








2 replies on “TLC-Thin Layer Chromatography”
I every time spent my half an hour to read this website’s articles everyday
along with a cup of coffee.
You should be very fond of Chemistry to do that! 😉